Clinical highlights – March 2023
Alzheimer's disease1
Alzheimer’s disease (AD) is a genetic and sporadic neurodegenerative disease that causes an amnestic cognitive impairment in its prototypical presentation and non-amnestic cognitive impairment in its less common variants. Biologically it is defined by the presence of β-amyloid-containing plagues and tau-containing neurofibrillary tangles. The severity of cognitive impairment in patients with AD varies. Dementia of gradual onset and ongoing progression with prominent amnestic symptoms and signs is the prototypical clinical phenotype of AD.
The epidemiology of AD is intertwined with that of all-cause dementia. The prevalence of all-cause dementia is expected to increase from 50 million people in 2010 to 113 million by 2050 worldwide.
Although there are no proved pharmacological nor non-pharmacological approaches for the prevention of cognitive impairment due to AD, there are grounds for optimism that multidimensional interventions that involve exercise, lifestyle changes and cognitive stimulation, combined with focused attention on other modifiable behaviours or conditions, might delay the onset of overt cognitive impairment.
Relevance of eye tracking inAlzheimer's disease
There is as yet no cure for AD. When a disease-modifying therapy will become available, it will be essential to administer this treatment in the very earliest stages of the disease, before pathological changes in the brain are widespread. Therefore, identifying the presence of AD in the pre-dementia ‘prodromal’ or even the ‘preclinical’ phase is essential. Eye-tracking has the potential to deliver biomarkers for the early diagnosis and monitoring of the disease. Please find hereafter 2 recent clinical papers showing promising results
Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment
Researchers have shown results confirming that the latency and the error rate in the antisaccade task are promising biomarkers for dementia. Given that people with MCI are more likely to develop dementia due to AD than cognitively healthy adults, and in particular that people with amnesic Mild Cognitive Impairment (aMCI) are at the highest risk of progressing to a full dementia syndrome, this may also offer additional prognostic biomarkers for predicting which people with a diagnosis of MCI are more likely to progress to dementia due to AD.
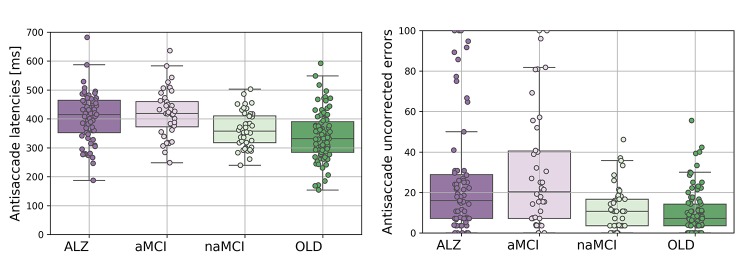
” The mean latencies of aMCI group were significantly longer than the naMCI group (t(84)=3.607;p=.001; d=.79), and the CP group (t(129)=5.116;p<.0005; d=.90).
…
Critically, the aMCI group (N=42; mean proportion=30; SD=30; 95% CI=21-39) generated a higher proportion of antisaccade errors compared to the naMCI group (N=46; mean proportion=12; SD=11; 95% CI=9-16; χ2(1)=11.774; p=.001) and the CP group (χ2(1)=21.806; p<.0005). “
Wilcockson et al. in Aging 2019
Saccadic Eye Movement in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis
In a large review comprising a total sample size of 35 studies with 2435 subjects, 1252 controls, and 1183 patients (386 MCI and 797 AD patients), researchers from South Korea suggested that the prosaccade and antisaccade latency and error rate could be used to distinguish patients from controls and MCI from AD within patient groups.
“… during antisaccades in the gap condition, when patients were compared with controls, patients with AD had significantly more errors than patients with MCI, and these errors, corresponding to a large effect size in AD and a moderate effect size in MCI, could be used to differentiate the two groups.”
Opwonya et al. in NeuroPsychol Rev. 202